GENERIC. FLEXIBLE. ESTABLISHED.
Write DAW.
Fight kidney, liver, and heart transplant rejection with GENGRAF®

Generic immunosuppression for kidney, liver, and heart transplantation1,2
- An established modified cyclosporine to help prevent transplant rejection
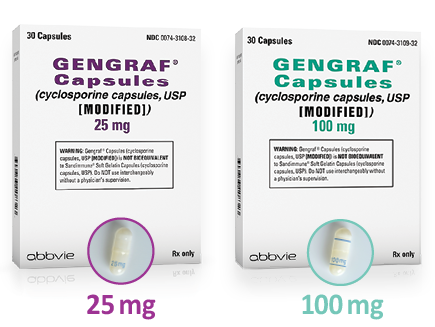
Capsules not actual size
Flexible dosing options
Available in:
- 25-mg capsules
- 100-mg capsules
- 100-mg oral solution

Depend on DAW to ensure your patients receive the GENGRAF that you prescribe3
- 98% of your patients receive GENGRAF when you write “Dispense as Written” (DAW)3
- As you may know, additional blood testing is required when switching to other modified cyclosporines4
- Simplify DAW: Download an electronic health record (EHR) quick reference guide here
IMPORTANT SAFETY INFORMATION1,2
WARNING
Only physicians experienced in the management of systemic immunosuppressive therapy for the indicated disease should prescribe GENGRAF. At doses used in solid organ transplantation, only physicians experienced in immunosuppressive therapy and management of organ transplant recipients should prescribe GENGRAF. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient.
GENGRAF, a systemic immunosuppressant, may increase susceptibility to infection and the development of neoplasia. In kidney, liver, and heart transplant patients, GENGRAF may be administered with other immunosuppressive agents. Increased susceptibility to infection and the possible development of lymphoma and other neoplasms may result from the increase in the degree of immunosuppression in transplant patients.
GENGRAF Capsules (cyclosporine capsules, USP [MODIFIED]) and GENGRAF Oral Solution (cyclosporine oral solution, USP [MODIFIED]) have increased bioavailability in comparison to Sandimmune®* Soft Gelatin Capsules (cyclosporine capsules, USP) and Sandimmune® Oral Solution (cyclosporine oral solution, USP), respectively. GENGRAF and Sandimmune are not bioequivalent and cannot be used interchangeably without physician supervision. For a given trough concentration, cyclosporine exposure will be greater with GENGRAF than with Sandimmune. If a patient who is receiving exceptionally high doses of Sandimmune is converted to GENGRAF, particular caution should be exercised. Cyclosporine blood concentrations should be monitored in transplant patients taking GENGRAF to avoid toxicity due to high concentrations. Dose adjustments should be made in transplant patients to minimize possible organ rejection due to low concentrations. Comparison of blood concentrations in the published literature with blood concentrations obtained using current assays must be done with detailed knowledge of the assay methods employed.
Cyclosporine, the active ingredient in GENGRAF, in recommended dosages, can cause systemic hypertension and nephrotoxicity. The risk increases with increasing dose and duration of cyclosporine therapy. Renal dysfunction, including structural kidney damage, is a potential consequence of cyclosporine; therefore, renal function must be monitored during therapy.
CONTRAINDICATIONS
GENGRAF is contraindicated in patients with a hypersensitivity to cyclosporine or to any of the ingredients of the formulation.
WARNINGS
GENGRAF can cause nephrotoxicity and hepatotoxicity. The risk increases with increasing doses of GENGRAF; therefore, renal and hepatic function must be monitored during therapy. Due to the potential for additive or synergistic impairment of renal function, caution should be taken when using GENGRAF with other nephrotoxic drugs or other drugs that impair renal function.
Patients receiving GENGRAF require frequent monitoring of serum creatinine. Elderly patients should be monitored with particular care, since decreases in renal function occur with age.
Nephrotoxicity: GENGRAF can cause nephrotoxicity and hepatotoxicity when used in high doses. It is not unusual for serum creatinine and BUN levels to be elevated during cyclosporine therapy. These elevations in renal transplant patients do not necessarily indicate rejection, and each patient must be fully evaluated before dosage adjustment is initiated.
Thrombotic Microangiopathy: Occasionally patients have developed a syndrome of thrombocytopenia and microangiopathic hemolytic anemia, which may result in graft failure.
Hyperkalemia: Significant hyperkalemia (sometimes associated with hyperchloremic metabolic acidosis) and hyperuricemia have been seen occasionally in individual patients.
Hepatotoxicity: Cases of hepatotoxicity and liver injury, including cholestasis, jaundice, hepatitis, and liver failure, have been reported in patients treated with cyclosporine. In some cases, mainly in transplant patients, fatal outcomes have been reported.
Malignancies: Patients receiving cyclosporine are at increased risk for the development of lymphomas and other malignancies, particularly those of the skin. Patients taking cyclosporine should be warned to avoid excess exposure to ultraviolet light. Some malignancies may be fatal.
Serious Infections: Patients receiving GENGRAF are at increased risk of developing bacterial, viral, fungal, and protozoal infections, including opportunistic infections. These infections may lead to serious, including fatal, outcomes.
Polyoma Virus Infections: Patients receiving GENGRAF are at increased risk for opportunistic infections, including polyoma virus infections. Polyoma virus infections in transplant patients may have serious and sometimes fatal outcomes. These include cases of JC virus–associated progressive multifocal leukoencephalopathy (PML) and polyoma virus–associated nephropathy (PVAN), especially due to BK virus infection.
Neurotoxicity: There have been reports of convulsions in adult and pediatric patients receiving cyclosporine, particularly in combination with high-dose methylprednisolone.
Encephalopathy, including posterior reversible encephalopathy syndrome (PRES), has been described both in postmarketing reports and in the literature.
Special Excipients – Alcohol (Ethanol): The alcohol content of GENGRAF Capsules should be taken into account when given to patients in whom alcohol intake should be avoided or minimized, e.g., pregnant or breastfeeding women, patients presenting with liver disease or epilepsy, alcoholic patients, or pediatric patients.
PRECAUTIONS
Hypertension: Hypertension is a common side effect of cyclosporine therapy that may persist; antihypertensive therapy may be required. However, since cyclosporine may cause hyperkalemia, potassium-sparing diuretics should not be used.
Vaccination: During treatment with cyclosporine, vaccination may be less effective; the use of live attenuated vaccines should be avoided.
Drug Interactions: A number of drugs are well substantiated to interact with cyclosporine. In addition, concomitant use of NSAIDs with cyclosporine, particularly in the setting of dehydration, may potentiate renal dysfunction. Caution should be exercised when using other drugs which are known to impair renal function, and close monitoring of renal function (in particular serum creatinine) should be performed.
CYP3A inhibitors may result in increased cyclosporine concentrations, while CYP3A inducers may decrease cyclosporine concentrations. Monitoring of concentrations and appropriate adjustment of GENGRAF dosage should be made as needed with concomitant use of either CYP3A inhibitors or inducers.
Cyclosporine may reduce the clearance of digoxin, colchicine, prednisolone, HMG-CoA reductase inhibitors (statins), aliskiren, bosentan, dabigatran, repaglinide, NSAIDs, sirolimus, etoposide, and other drugs.
Severe digitalis toxicity has been seen within days of starting cyclosporine in several patients taking digoxin.
Literature and postmarketing cases of myotoxicity, including muscle pain and weakness, myositis, and rhabdomyolysis, have been reported with concomitant administration of cyclosporine with lovastatin, simvastatin, atorvastatin, pravastatin, and, rarely, fluvastatin. The coadministration of cyclosporine with aliskiren is not recommended. Coadministration of cyclosporine with bosentan or with dabigatran should be avoided. Cyclosporine should not be used with potassium-sparing diuretics because hyperkalemia can occur. Cyclosporine may increase the plasma concentrations of repaglinide and thereby increase the risk of hypoglycemia.
There have been reports of a serious drug interaction between cyclosporine and the herbal dietary supplement St. John’s Wort that resulted in subtherapeutic levels of cyclosporine, rejection of transplanted organs, and graft loss. Grapefruit and grapefruit juice affect metabolism, increasing blood concentration of cyclosporine, and should be avoided.
Please see the full Prescribing Information for a listing of established and other potentially significant drug interactions.
Pregnancy: GENGRAF should not be used during pregnancy unless the potential benefit to the mother justifies the potential risk to the fetus. The alcohol content of GENGRAF Capsules should also be taken into account in pregnant women.
Nursing Mothers: Cyclosporine passes into breast milk. Because of the potential for serious adverse drug reactions in nursing infants from GENGRAF, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use: Elderly patients are more likely to develop systolic hypertension on therapy and are more likely to show serum creatinine rises ≥50% above the baseline after 3 to 4 months of therapy. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range.
ADVERSE REACTIONS
Kidney, Liver, and Heart Transplantation: The principal adverse reactions of cyclosporine therapy are renal dysfunction, tremor, hirsutism, hypertension, and gum hyperplasia.
Hypertension: Usually mild to moderate, hypertension may occur in approximately 50% of patients following renal transplantation and in most cardiac transplant patients.
Glomerular Capillary Thrombosis: Glomerular capillary thrombosis has been found in patients treated with cyclosporine and may progress to graft failure.
Hypomagnesemia: Hypomagnesemia has been reported in some, but not all, patients exhibiting convulsions while on cyclosporine therapy.
INDICATION1,2
Kidney, Liver, and Heart Transplantation: GENGRAF® Capsules (cyclosporine capsules, USP [MODIFIED]) and GENGRAF® Oral Solution (cyclosporine oral solution, USP [MODIFIED]) are indicated for the prophylaxis of organ rejection in kidney, liver, and heart allogeneic transplants. Cyclosporine has been used in combination with azathioprine and corticosteroids.
*Sandimmune® is a registered trademark of Novartis Pharmaceuticals Corporation.
References:
1. GENGRAF Capsules [package insert]. North Chicago, IL: AbbVie Inc.
2. GENGRAF Oral Solution [package insert]. North Chicago, IL: AbbVie Inc.
3. Data on File. 2017 DMD Pharmacy Audit.
4. Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77(4):299-311. doi:10.1038/ki.2009.377
Please see full Prescribing Information about GENGRAF Capsules.
Please see full Prescribing Information about GENGRAF Oral Solution.
If you have any questions about AbbVie's Gengraf.com website that have not been answered,
click here.
This website and the information contained herein is intended for use by U.S. physicians only and is provided for informational purposes only.
![Gengraf Capsules (cyclosporine capsules, USP [MODIFIED])](/Content/img/Gengraf_logo_360.png)